Aging is defined as a progressive loss of physical integrity, functionality, and increased vulnerability to illness and death that begins on the cellular level.
While aging itself isn’t a disease, the aging process represents a major risk factor for several chronic diseases and conditions, including frailty and lack of resilience.
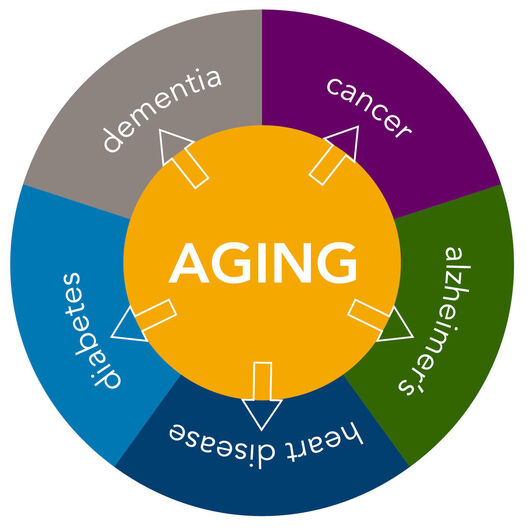
Research on the biology of aging has accelerated rapidly in the last two decades. Geroscience, a new branch of longevity science, seeks to address the biology of aging and the biology of age-related diseases together.
Aging is a predominant risk factor for most common chronic diseases that limit health span: cardiovascular disease, diabetes, cancer, metabolic syndrome, dementia, and Alzheimer’s disease, to name a few.
Accumulated cellular waste, signaling errors, imperfect repairs, and cell damage all contribute to the symptoms of aging and ultimately lead to the development of age-related diseases that eventually kill us.
Understanding cell dysfunction and how it affects our bodies is the key to understanding why we age.
This post explores what longevity scientists call the Ten Hallmarks of Aging and how they each contribute to the process.
There’s a bit of biochemistry here, but don’t worry, we’ll decipher it further in workshop #1, on October 22, 2022, of THE BIG REWIND.
Reserve your spot today.

mitochondrial dysfunction
- Mitochondria are the power plants of our cells. They produce the energy that our cells need to stay alive and function properly.
- Cells contain hundreds to thousands of mitochondria on average.
- The older we get, the more our mitochondria become damaged and dysfunctional.
- Mitochondria become damaged in a variety of ways during our lifetime, such as mutations
- in their DNA when they divide, by free radicals, and due to epigenetic changes.
- When mitochondria are damaged, cells don’t have enough energy to properly function and maintain themselves.
- Also, damaged mitochondria send disruptive signals to the cell, further disrupting proper cellular functioning.
- All of this contributes to aging.
Cellular Senescence
Senescent cells are sometimes called “zombie” cells because they are damaged cells that should have died but stay alive.
Senescent cells secrete inflammatory substances that damage the healthy surrounding cells, further contributing to inflammation and aging.
When we get older, these senescent cells accumulate in the skin, contributing to sagging of the skin and wrinkles.
In the joints, senescent cells damage the cartilage, contributing to osteoarthritis.
Senescent cells in the blood vessel walls lead to stiffer blood vessels which are more prone to breaking and accumulating plaque.
Altered Cellular Communication
- During aging, the environment our cells live in changes: it becomes pro-inflammatory, pro-aging, and damaging. This makes our cells age faster, leading to a vicious cycle as damaged cells secrete harmful substances that damage healthy cells.
- When we get older, increasingly higher levels of destructive substances can be found in our bloodstream and cellular fluids.
- Examples of such substances are proinflammatory factors, proteins, peptides, metabolites, and hormones that damage our cells and accelerate aging.
- These circulating substances promote low-grade, systemic age-related inflammation also called “inflammaging”.
- A dysregulated microbiome, a leaky gut, an aging immune system, and chronic pathogens like viruses can all contribute to altered cell communication.

Epigenetic Alterations
The epigenome is the complex machinery that determines how active each of our genes are. You can look at the epigenome as an on-off switch for genes.
The epigenome is very important for gene expression (which genes are active or inactive), and thus cellular function as a whole.
The problem is that during aging the epigenome becomes increasingly dysregulated.
Liver-specific genes are switched on in brain cells, stomach genes are switched on in muscle cells, and so on.
Genes that need to be turned off are turned on (like cancer-promoting genes), and genes that need to be turned on are switched off, such as genes that repair or protect our cells.
Generally, we see that our DNA becomes more demethylated (there are less methyl groups sticking on the DNA which would normally prevent the DNA to be translated into protein). As a result, gene transcription is less suppressed in many areas.
This leads to specific genes becoming active that should not be active, like cancer-promoting genes.
Genomic Instability
- During our lifespan our DNA becomes damaged, contributing to many problems given DNA contains all the instructions to build and maintain our body.
During aging, our DNA becomes more and more damaged, contributing to the aging process.
DNA can become damaged in two main ways:
- Damage from the outside: This kind of damage is caused by external factors, like physical (e.g. UV light), chemical (e.g. specific drugs, substances in cigarette smoke, toxic compounds) and biological damage (e.g. viruses).
- Damage from the inside: This kind of DNA damage is caused by internal processes, such as replication errors when the DNA is copied, free radicals produced by cellular metabolism, spontaneous chemical reactions, a dysregulated epigenome, etc.
- These actions lead to all kinds of DNA damage, such as mutations, DNA strand breaks, chromosomes that disappear or get rearranged, telomere attrition and shortening, and more.
- Every day, in every cell, tens of thousands of insults damage the DNA, but most of them are repaired, fortunately. As we age, the genes that repair DNA can get overwhelmed.

Telomere Shortening
Telomeres are short pieces as the end of our DNA strands.
You can compare telomeres to the caps on our shoelaces that prevent the laces from raveling out.
With every cell division, telomeres become shorter. When they are too short, cells stop dividing. These cells cannot continue supporting and forming our tissues properly.
Telomere length shortens with age. Progressive shortening of telomeres leads to senescence, apoptosis (cell death), or oncogenic transformation of somatic cells, affecting the health and lifespan of an individual. Shorter telomeres have been associated with increased incidence of diseases and poor survival.
The rate of telomere shortening can be either increased or decreased by specific lifestyle factors.
Better choice of diet and activities has great potential to reduce the rate of telomere shortening or at least prevent excessive telomere attrition, leading to delayed onset of age-associated diseases and increased lifespan.

Loss of Proteostasis
Proteins are the building blocks and workhorses of our cells.
Each cell contains many millions of proteins. Proteins are continuously broken down and built up.
However, this process is not perfect: some proteins are not broken down and keep lingering around in the cell.
They clump together and start to
accumulate in and around the cells, causing the function of the cells to deteriorate, all contributing to the process we call aging.
“Proteostasis” refers to “protein homeostasis”.
Homeostasis is the delicate, healthy balance that all cells strive for to stay alive and function properly.
With age, proteostasis deteriorates.

Deregulated Nutrient Sensing
During aging, important metabolic pathways become more and more dysregulated. These pathways regulate how our cells respond to nutrition.
When our cells become less tuned to nutrient signals, it can result in reduced energy and metabolic dysfunction as we age.
The four pathways of nutrient-sensing regulate metabolism and influence aging. The four associated key protein groups are IGF-1, mTOR, sirtuins, and AMPK. We call these proteins “nutrient-sensing” because nutrient levels influence their activity. We will talk more about the impact of these proteins and what we can do about them in THE BIG REWIND workshop #2 on November 19, 2022.
It’s important to understand how the western diet with an overabundance of fast sugars, animal proteins, and unhealthy fats leads to an overactivation of these nutrient-sensing pathways and accelerates aging.

STEM CELL EXHAUSTION
- Stem cells are rare cells, scattered around in the body, that produce new, differentiated cells.
- Stem cells are very important and very powerful. In fact, your whole body was created out of one super stem cell, namely the fertilized egg cell that nestled itself in the womb of your mother.
- Our body is continuously rebuilt and replenished with new cells that derive from stem cells.
- When we get older, our stem cells become dysfunctional or they die off. This leads to our tissues being far less replenished with new, healthy cells.
- Additionally, as we age, some dysfunctional stem cells take over the existing stem cell pool. These stem cells are dysfunctional because they don’t maintain the tissues properly, but they reproduce faster than the normal stem cells, out-competing them.
Crosslinking
Cross-linking, (also known as glycosylation) attributes aging to chemical changes that happen gradually as proteins, structural molecules, and DNA develops detrimental chemical bonds (aka cross-links) to each other.
Cross-linking issues arise when glucose binds to protein. This process occurs under the presence of oxygen, and as we age there are increased odds that oxygen comes in contact with glucose and protein to activate the cross-linking transition.
This is somewhat similar to how apple slices (a glucose-rich food) will gradually turn yellow and brown as they are exposed to oxygen in the air.
Cross-linking of proteins may also play a role in the hardening of collagen and cardiac enlargement, increasing the risk for cardiac arrest.
Cross-linking is also associated with stiffening of blood vessel walls, delayed wound healing, reduced joint mobility, and changes in the lens of the eye.
In addition to these potentially serious implications, many believe that cross-linking is responsible for age-related skin changes including wrinkles and reduced elasticity.
As we grow older, sugar-derived bonds, or crosslinks, are formed between the proteins that make up our tissues, making tissues more stiff. Stiffer tissue is less capable of performing its function than soft, supple tissue, especially in blood vessels, the lungs and the skin.

summary
Aging changes occur in all of the body’s cells, tissues, and organs, and these changes affect the functioning of all body systems.
While your chronological age (how many birthdays you’ve had), will increase at a set rate as the years pass, your biological age, the measurement of your cellular age based on various biomarkers — can change due to how you choose to live your life.
Your biological age reflects a combination of your genetics, accumulated lifestyle factors, and other determinants such as demographics, diet, and exercise habits.
Addressing aging as the biochemical event that it is can help you reduce inflammaging, slow the ten hallmarks of aging, and help keep you strong, flexible, energetic, and focused.
Now that we know why we age. We’ll take a deeper dive into what we can do about it in our second in person workshop with Dr, Rahul Kapur on Saturday, November 19, 2022.
Our next blog in this series will detail actionable steps you can take today for a younger tomorrow! Scheduled blog release date: October 22, 2022.

The mechanism of aging all boils down to keeping our mitochondria and DNA clean, healthy, and functional.
In addition to eating healthy, moving regularly, and staying connected in your community, new scientific discoveries in longevity can help us not only live longer but better, healthier, and with more joy!
Join Tina Sprikle for THE BIG REWIND, a 3-part series exploring the reasons why we age and what we can do about it.
workshop #1.
why we age
SATURDAY, OCTOBER 22, 2022
Take a deep dive into the biochemistry of aging, and longevity science, to learn how you can not only live longer but live better!

workshop#2.
what we can do
Saturday, November 12, 2022
The importance of nutrition, meal timing, exercise, targeted supplementation, and peptide therapy for slowing down and reversing aging with special guest Dr. Rahul Kapur.

workshop #3. ask the surgeon
Saturday, January 14, 2023
You’re eating right, exercising and working your anti-aging protocol but still want a fresher face in the mirror. Join special guest Dr. Regina Nouhan as she answers your questions about options for facial cosmetic surgery.
Each 90-minute workshop provides participants with:
- The latest longevity research education
- Actionable steps to improve your health, slow and or reverse aging
- Workshop handout and Ebook
- Resources links and service providers
- Video replay
- Optional follow-up resource texts
until we get this party started why we age - 10.22.2022
Questions?
I’m always a text or email away.
Email me at tina@tinasprinkle.com or send me a text at 913 963 8546.

